IPN Neuroplastogen enters Phase IB Clinical Trial
We are thrilled to share an exciting milestone in the translation of psychedelic-related therapeutics: Delix Therapeutics has dosed the first patient in a clinical trial for DLX-001, a non-hallucinogenic neuroplastogen designed to treat depression. They are currently in phase Ib, and hope to move on to phase II soon. This drug originated from research conducted by David E. Olson’s team at the IPN, marking a significant step from foundational discoveries to potential real-world impact.
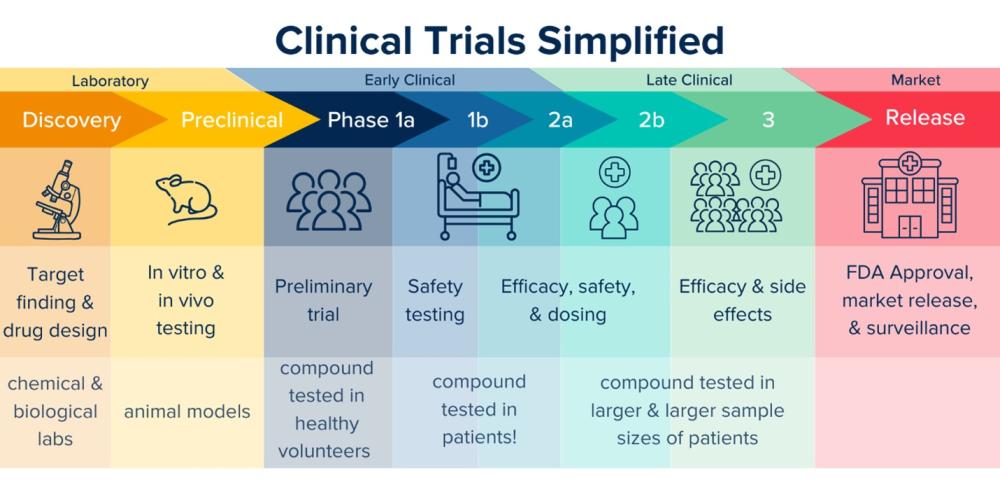
At the IPN, our mission has always been to advance the science of neuroplasticity-promoting compounds, particularly those that offer the therapeutic benefits of psychedelics without the hallucinogenic effects. That work led to the synthesis DLX-001 and additional insights into the ability of these compounds to promote structural and functional neural plasticity, laying the groundwork for this new generation of neurotherapeutics.
Seeing these discoveries move from the lab to clinical trials is a testament to the power of interdisciplinary collaboration and rigorous research. As we continue to explore and develop next-generation treatments, we look forward to more breakthroughs that expand access to safe, effective mental health solutions.
Stay tuned for more updates as this research progresses into phase II clinical trials!
Disclaimer:
- The clinical trials mentioned in this article are being conducted by Delix Therapeutics, not the IPN. The IPN cannot help you access these clinical trials.
- DLX-001 is not currently available to the public at the time of this publishing.
- The contents of our website are for informational purposes only. This website is not for emergency or crisis help. Our programs are not intended to provide mental health diagnoses, counseling, or treatment. You should always seek advice from your physician or other qualified healthcare providers if you are experiencing symptoms of depression, anxiety, or other mental health conditions.
